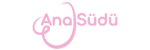Drug instructions for use during lactation
Although the modern healthcare system has existed for over 100 years, and breastfeeding mothers have been part of human history throughout all ages, only a limited number of books and scientific publications on medications, including drugs, and human lactation have been published to date. Information related to lactation in medication instructions is particularly scarce. The first printed book dedicated to the interaction between medicinal products and human lactation was published in 1988, and its updated edition appeared in 1996.
In the book Drugs and Human Lactation by P. N. Bennett, 205 medicinal products with the potential to affect human lactation were examined. It was established that 87% of these medications are permitted for use by mothers during the breastfeeding period. The remaining 10% of the studied drugs fall into a risk category. That is, they belong to a potentially hazardous zone: depending on the mother’s health status, diagnosis, and the recommendations of the attending physician, the medication may be taken or, conversely, avoided. In other words, the drug may or may not cause complications in the mother. There is no unequivocal consensus regarding the safety of medications within this category.
Mothers should strictly avoid using the remaining 3% of medications during the lactation period. According to the data presented above, only a tiny proportion of existing drugs are contraindicated for breastfeeding women. The remaining medications can be taken without significant issues, provided they are used under the supervision of a treating physician. In the postpartum period, that is, during breastfeeding, a mother’s use of medications also follows a certain principle: if a drug can be safely used during pregnancy, it can generally also be used during breastfeeding. However, this rule applies in only one direction: medications approved for use during pregnancy are usually acceptable during breastfeeding, but drugs considered safe during lactation are not necessarily safe during pregnancy. When questions arise regarding medication use during lactation, and the physician lacks sufficient expertise in this area, one may reasonably ask the following question:
“Is the medication you have prescribed suitable for use during pregnancy?”
If a medication is approved for use during pregnancy and, moreover, is prescribed to young children, then its use both during pregnancy and throughout the breastfeeding period can be considered normal and safe.
In his report, Philip O. Anderson draws attention to a crucial point: mothers often see a warning in the patient information leaflet of a physician-prescribed medication stating that its use is contraindicated during pregnancy and lactation. In reality, however, this does not always reflect the true situation. In many cases, this statement is merely a disclaimer added by pharmaceutical manufacturers as a precautionary measure to protect themselves and limit liability toward patients. In other words, such a statement means the medication might be suitable for a breastfeeding mother.
For the information in medication leaflets regarding use by breastfeeding women to be fully reliable, pharmaceutical companies would need to conduct long-term, large-scale, and costly studies. To avoid these expenses, companies often choose, as a precaution, to indicate contraindications for pregnant and breastfeeding women in the instructions. The presence of such a warning is not necessarily based on scientific research; rather, it represents a safety measure on the part of the manufacturer.
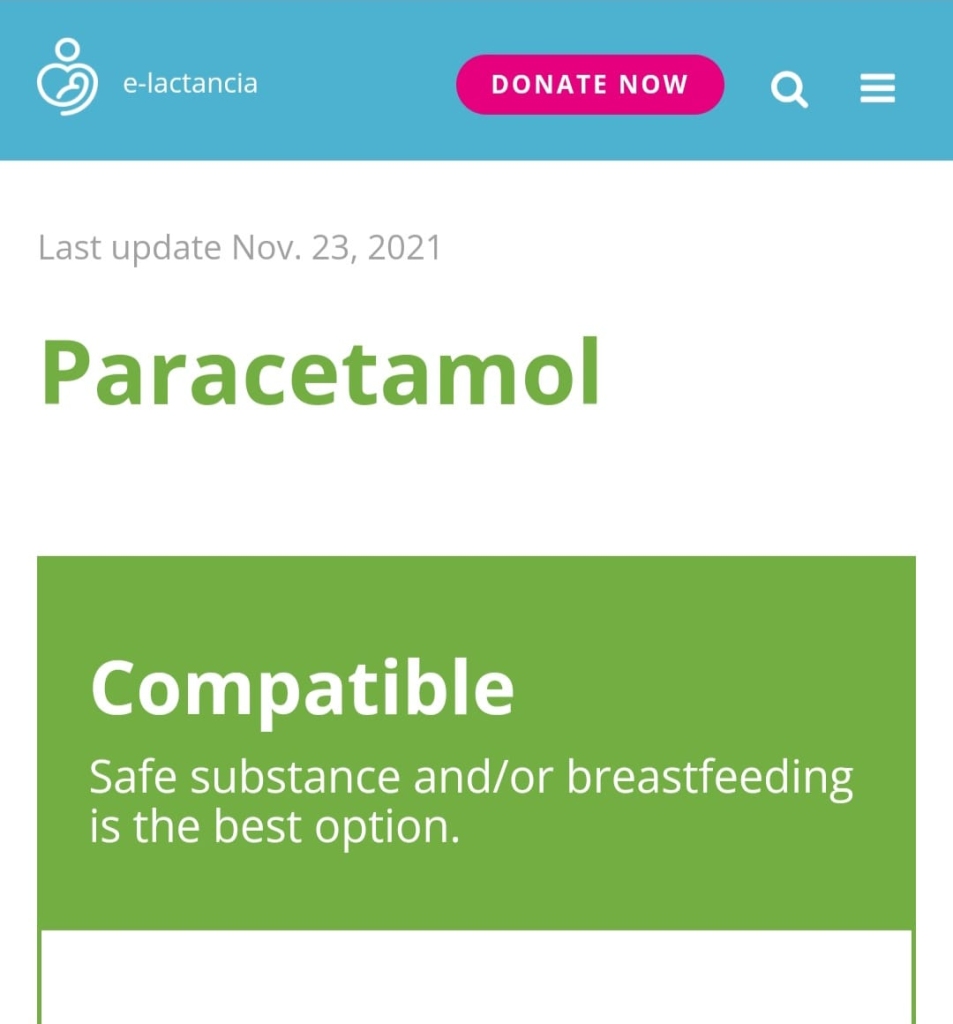
There are international sources of information, updated every few years in electronic form or as books, that focus on the study of medication safety. Among them are the websites E-lactancia and LactMed. By visiting these resources, mothers can enter either the brand name of a medication or the name of its active substance into the search field and determine whether it is compatible with breastfeeding.
What is E-Lactancia?
Both healthcare professionals and mothers can use the international drug database E-Lactancia, which helps guide decisions regarding medication use. The initiative to create this website came from pediatricians and pharmacologists. The resource, whose aim is to help breastfeeding mothers continue breastfeeding without harm to milk production or the child’s health when treatment is necessary, was developed on the basis of evidence-based data provided by specialists from various medical fields around the world and, over time, has become a global source of information. No special medical knowledge is required to use the site: it is user-friendly and does not even require knowledge of a foreign language.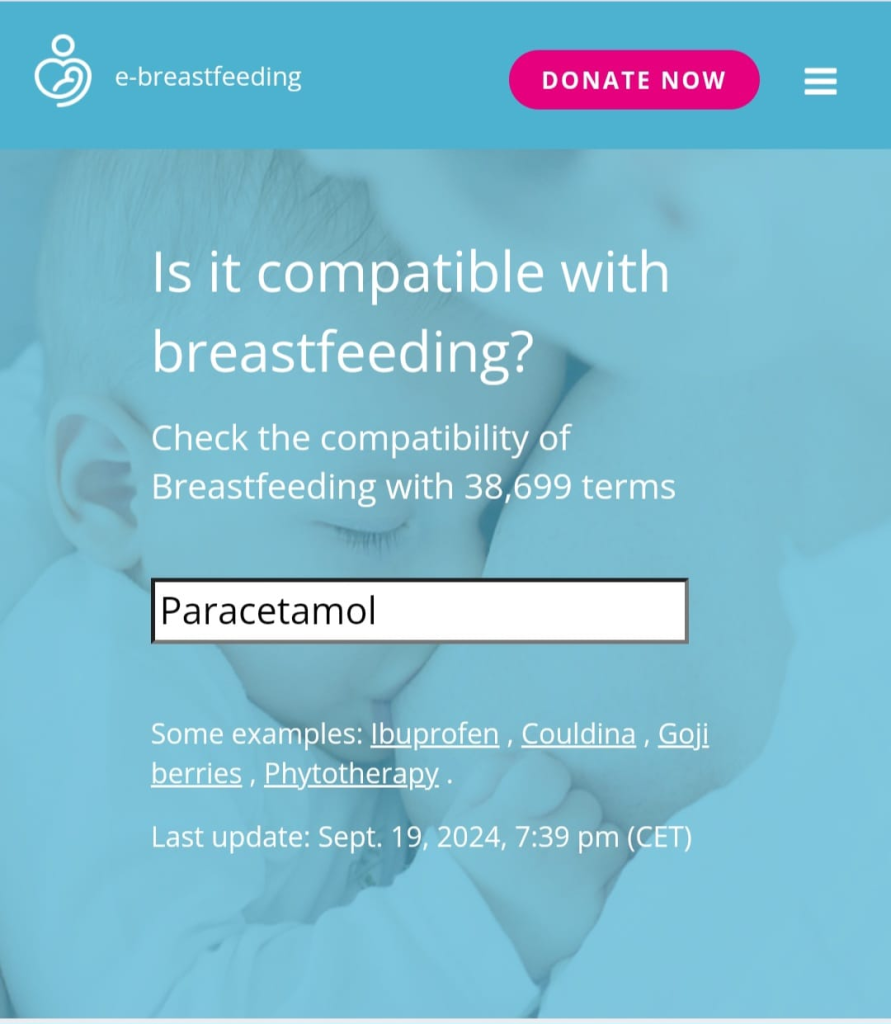

Author: Saltanat Zulfugarova
Breastfeeding Consultant